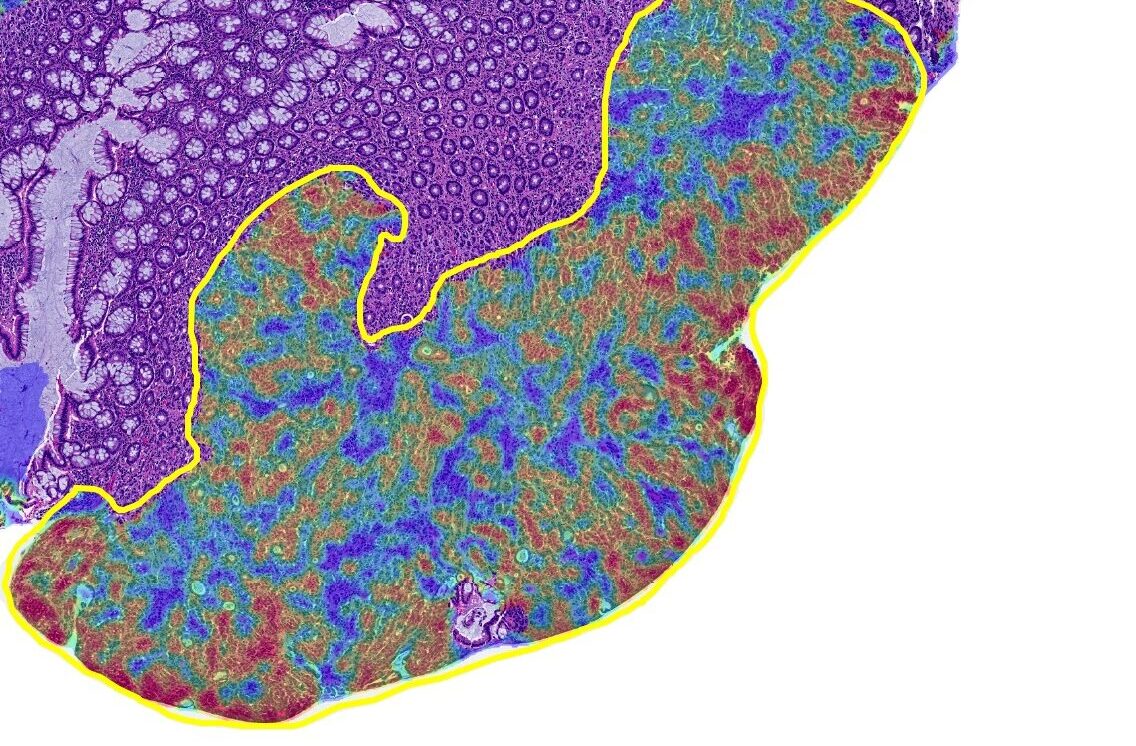
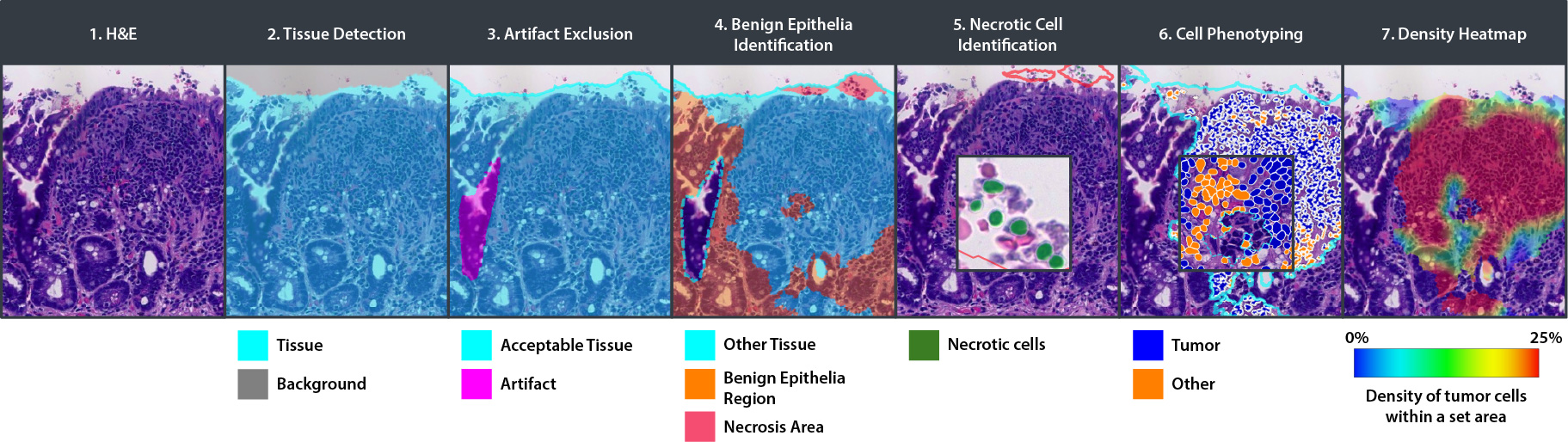
CRC Macrodissect AI is an AI-powered tool that quantifies tumor content and guides ROI selection to enhance macrodissection workflows and downstream molecular analysis in colorectal carcinoma.
Intended Use
Research Use Only
Inputs
H&E whole slide images from primary and metastatic CRC resections, excisions, and/or core needle biopsies.
Key Outputs
- Tissue Detection
- Artifact Exclusion
- Benign Epithelial Identification
- Necrotic Cell Identification
- Cell Phenotyping
- Density Heatmap
- Percent tumor content for whole slide image and dissection ROIs
Analytical Validation
CRC Macrodissect AI was validated on the Leica Aperio GT 450 (SVS format). Cellular level validation was performed on 20,782 cells/objects from both primary and metastatic CRC tissue previously unseen to the algorithm and sourced from an external site. The validation process focused on the classification of cells as either ‘tumor’ or ‘other,’ with the ground truth established through the consensus of annotations provided by five independent pathologists.

Clinical Validation
CRC Macrodissect AI was validated on the Leica Aperio GT 450 (SVS format).
280 externally sourced primary and metastatic colorectal cancer H&E images previously unseen by the algorithm were assessed for tumor content by five pathologists. After a four-week washout period, the five pathologists reviewed the same slides again, this time with the assistance of CRC Macrodissect AI. They had the option to agree with the algorithm’s analysis or provide their own tumor content estimations.
The intraclass correlation coefficient (ICC) was calculated using continuous tumor content data and Fleiss’ kappa was calculated after samples were dichotomized based on a 20% tumor content cut-off, a minimum requirement for most molecular tests. Both ICC and Fleiss’ kappa were measured before and after assistance from CRC Macrodissect AI.
CRC Macrodissect AI significantly increased the consistency and agreement of inter-pathologist tumor content reporting, demonstrating the algorithm’s ability to accurately quantify tumor content, standardize macrodissection workflows, and reduce the number of inadequately concentrated tests sent for downstream analysis.
Inter-Pathologist Agreement of Tumor Content Estimation With and Without CRC Macrodissect AI
File Formats:
- Non-proprietary (JPG, TIF, OME.TIFF, DICOM [DCM*])
- Leica (SVS, AFI, SCN, LIF)
- Hamamatsu (NDPI, NDPIS)
- Philips (iSyntax, i2Syntax)
- 3DHistech (MRXS)
- Nikon (ND2)
- Akoya (QPTIFF, component TIFF)
- Olympus / Evident (VSI)
- Zeiss (CZI)
- Ventana (BIF)
- KFBIO (KFB, KFBF)
*whole-slide images
CRC Macrodissect AI Brochure
Learn more about the automated workflow and benefits of CRC Macrodissect AI.
Submit the form below to view the requested document
HALO Clinical AI Solutions Flyer
Check out our flyer to learn more about HALO Clinical AI Solutions.
Submit the form below to view the requested document

Confidence in Results
CRC Macrodissect AI reliably quantifies tumor content for downstream molecular analysis, ensuring the quality of downstream test results.

Streamline Workflows and Save Resources
With automated tumor content analysis, you can streamline your ROI selection process and save time.

Auditable Process
Create an auditable macrodissection workflow, ensuring transparency, efficiency, and accuracy of molecular test results.
CRC Macrodissect AI bridges the gap between anatomic and molecular pathology by simplifying the macrodissection process. Pathologists need only use the intuitive annotation tools provided in HALO AP® to select areas of high tumor density for downstream analysis by following the easy-to-read heatmap. Tumor content results for annotated ROIs are updated in real time.
Reach out to learn more!
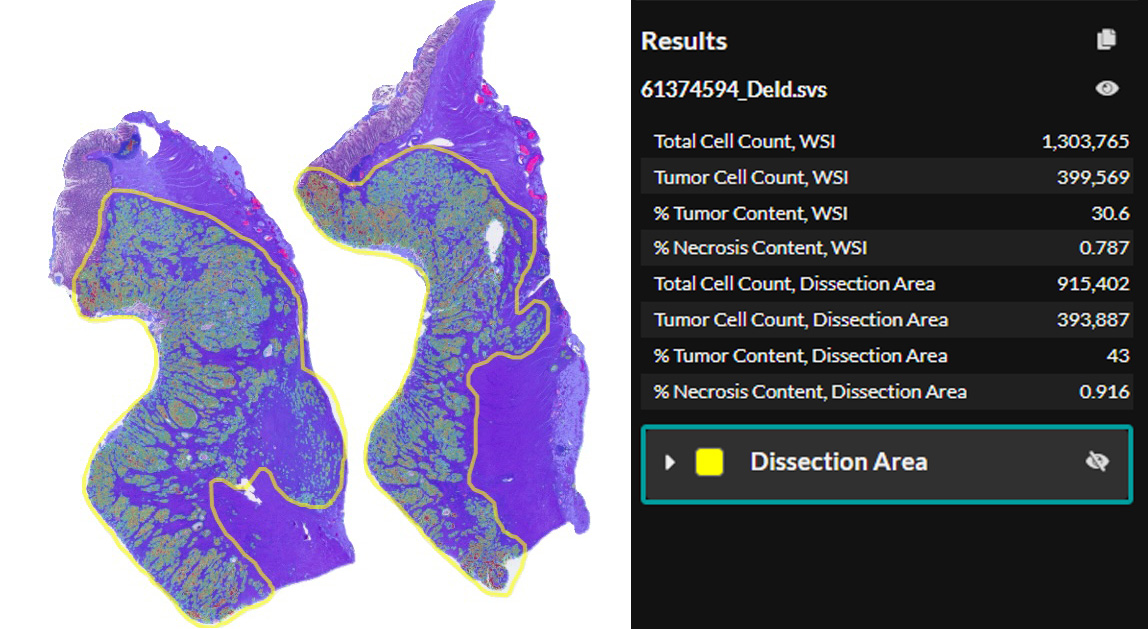
Macrodissection Reinvented
CRC Macrodissect AI enhances macrodissection workflows with precision and automation. H&E slides are scanned into HALO AP®, where CRC Macrodissect AI detects all tissue present on the slide, removing background glass and artifacts from the analysis. Benign epithelial and regions of necrosis are classified separately, and their cell count is added to the tumor content results. Cells are then phenotyped as either ‘tumor’ or ‘other’ cells. A detailed tumor density heatmap is generated, which assists pathologists in creating precise ROI annotations for downstream macrodissection.
Interested in learning more?
Schedule a call to see how CRC Macrodissect AI can meet your needs.
A Fully-Automated CRC Macrodissect AI Workflow
In addition to standardizing the evaluation of tumor content for traditional macrodissection workflows, CRC Macrodissect AI can be coupled with the Tissector automated macrodissection platform from our partner Xyall, creating an all-in-one solution that is auditable, precise, and more efficient than current methodologies. The result is a streamlined workflow that enhances accuracy and saves staff time and resources.
Macrodissect with Confidence
With a combined HALO Macrodissection Solutions and Xyall workflow, labs create a detailed audit trail for improved accuracy and consistency, including pre- and post-dissection images for each case.
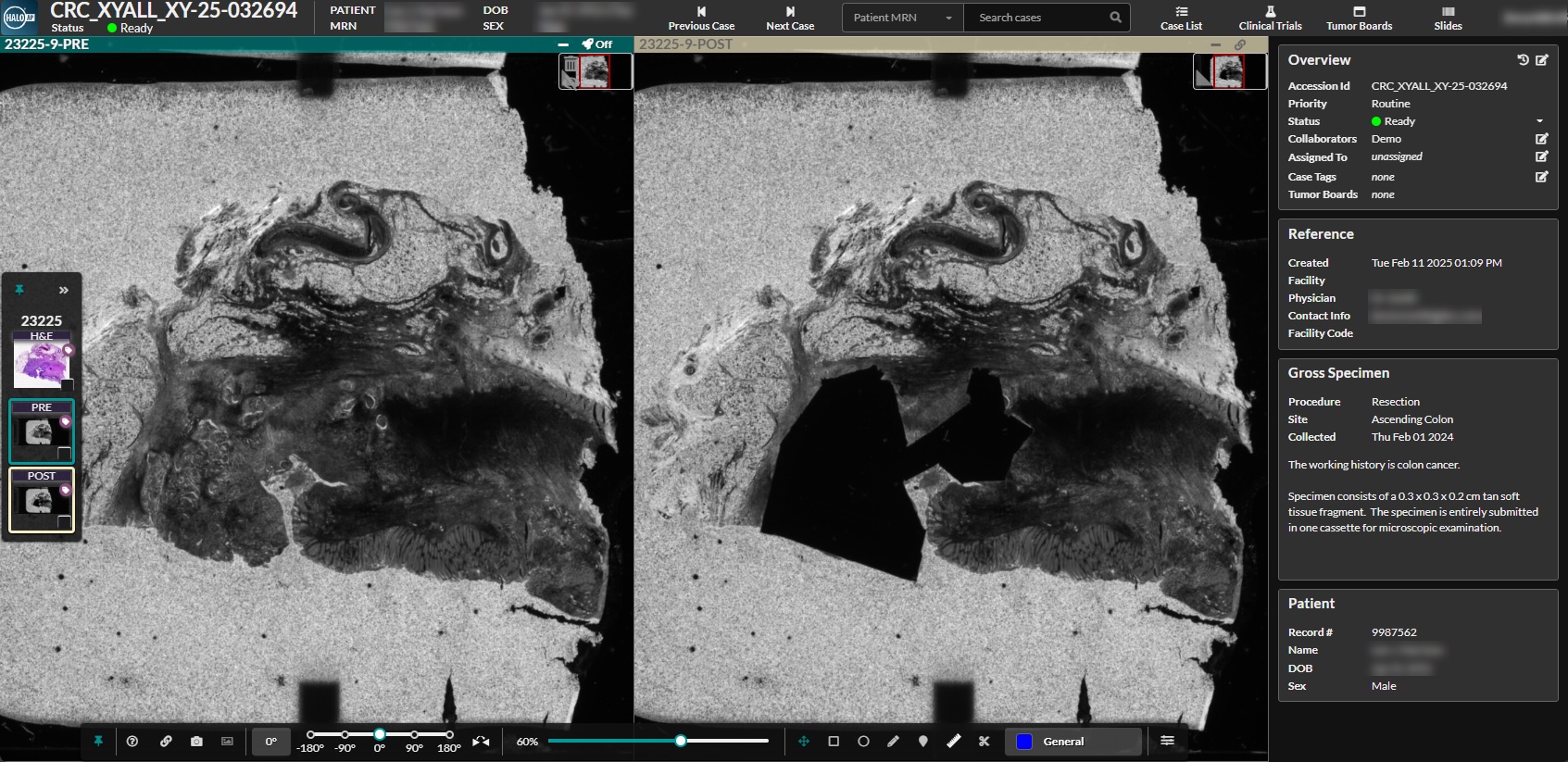
Bridging the Gap with an AI-Powered Workflow
By providing accurate and reliable tumor content quantification, CRC Macrodissect AI gives labs the ability to create a high-throughput, intuitive workflow that saves valuable lab personnel time and bridges the gap between anatomic and molecular pathology.
- Auditable
- Annotation guidance
- Tumor content quantification
- Highly precise macrodissection*
- Pre- and post-dissection images*
- Auditable
- Annotation guidance
- Tumor content quantification
- Highly precise macrodissection*
- Pre- and post-dissection images*

Seamless Deployment in HALO AP®
CRC Macrodissect AI is deployed and fully integrated into HALO AP®, the AI-powered, pathologist-driven platform for anatomic pathology workflows from Indica Labs.

Want to Learn More?
Fill out the form below to request a live demo of CRC Macrodissect AI or to learn more about our other clinical solutions. You can also drop us an email at info@indicalab.com
Regulatory Compliance
CRC Macrodissect AI is not a medical device in the EU/UK and is not intended to be used for diagnostic purposes. CRC Macrodissect AI is accessed via the HALO AP® enterprise digital pathology platform. CRC Macrodissect AI is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.